Welcome to a blog managed by the American Museum of Science and Energy Foundation. The purpose of this blog is to feature guest commentators on a variety of topics under the banners of Science and Energy.

Author: Katie Francis provides technical writing, editing, and project management for engineers and coaches engineering teams to become better technical writers.
Katie has nearly two decades of experience in engineering seismology, working predominantly on nuclear and hydroelectric projects. Her engineering work ranged from performing seismic hazard and other earthquake ground-motion-related analyses to developing nuclear quality assurance (NQA-1) programs and project management. As the founder of KF Consulting, Katie combines her engineering problem-solving competence with a passion for writing to create clear, engaging, and audience-focused documents that help companies succeed.
We are excited to share some of Katie’s experience and knowledge.
NUCLEAR POWER BASICS
How’d you do on your nuclear quiz? Are you a nuclear genius? If so, you can probably skip for
today, but I hope you don’t. I always want to hear your insights and perspectives. But alas, we
are all so busy…
If you didn’t score so well, the NUCLEAR POWER BASICS posts are especially for you. These
posts will focus on explaining the science behind nuclear energy, starting with topics like these:
• What is the source of nuclear energy?
• How is nuclear energy generated?
• How does a nuclear reactor work?
We’ll add more topics as we go. Today, we will start with the first.
What is the source of nuclear energy?
Atoms are the source of nuclear energy. They generate the heat used to produce electricity
when they are either split apart to result in new smaller (lighter) atoms or slammed together to
create bigger (heavier) atoms.
Atoms have a simple structure. They consist of a nucleus, or core, made of positively charged
protons and no charge neutrons. Negatively charged electrons circle the nucleus in orbits.
Below is a diagram of helium with the atomic symbol He. Helium has two protons and two
neutrons in its core and is orbited by two electrons.
And atoms are tiny. At an average of 100 picometers across – That’s one 10 billionth of a meter!
– atoms are smaller than the shortest wavelength of visible light at about 400 nanometers, or
four 10 millionths of a meter. And the nucleus of an atom is 10,000 times smaller than the atom
itself! Atoms are so small they cannot even be seen with a light microscope.
Why, you ask?
Because if we picture an atom like a speck of dust, visible light waves are on the order of the
size of a basketball. Light waves are simply too big to “see” an atom. Imaging atoms requires
specialized equipment, but we’ll reserve this discussion for another day.
For now, let’s get a handle on how tiny atoms are. Take a look at the edge of a standard piece
of printer or notebook paper. Google says the average thickness of a sheet of paper is about
0.1mm. That’s one ten thousandth of a meter. Considering that pencil lead is graphite (carbon)
with a diameter of about 140 picometers, you can line up 714,286 carbon atoms end-to-end
across the width of the paper’s edge by coating it with the graphite from your pencil.
Atoms are defined by the number of protons in the nucleus, called the atomic number. They
are organized on the periodic table, like the one below from the American Chemical Society.
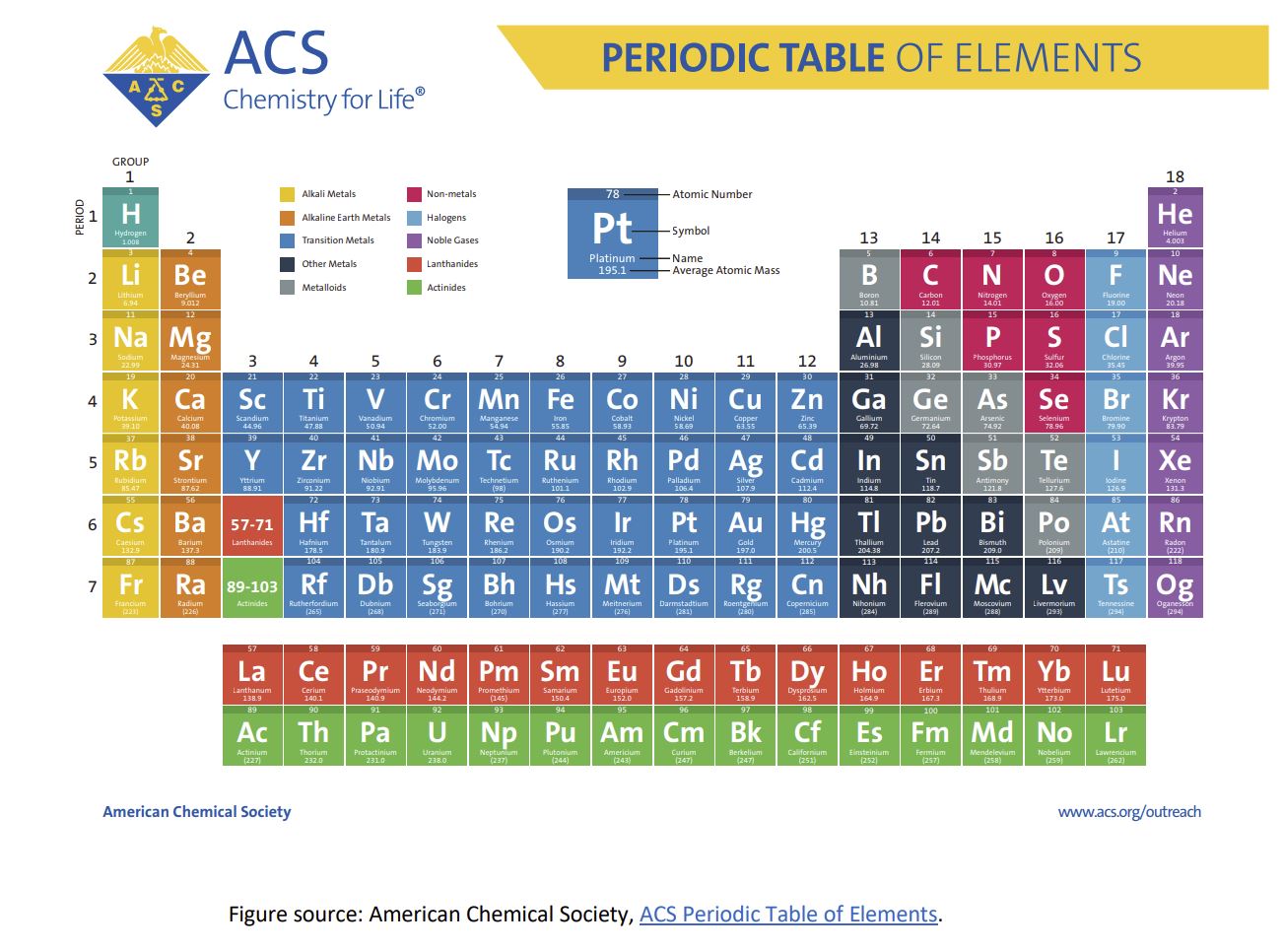
This basic periodic table is organized into blocks describing all of the atoms in our known
universe. If this is a new concept to you, pause for a moment and take it in. Everything in our
universe is made from a combination of these 118 atoms.
The table above is called a periodic table of the elements because atoms are the smallest
building blocks of a substance, and elements are substances that consist of a collection of only
one type of atom. So, don’t be confused if you hear “atom” and “element” used
interchangeably.
If we look at helium (He) in the table above (hint: top right corner), we see that helium has an
atomic number of 2, meaning it has two protons in its nucleus. It also has an average atomic
mass of 4. Atomic mass is the total mass of particles in an atom, approximately equivalent to
the number of protons and neutrons since the mass of electrons is negligible.
In all unbonded atoms (atoms that aren’t chemically combined with other atoms), the number
of electrons orbiting the nucleus equals the number of protons inside the nucleus. However,
different atoms of the same element can have different numbers of neutrons. We call atoms of
the same element with different numbers of neutrons isotopes of that element. We’ll discuss
isotopes throughout this series, starting with our next NUCLEAR POWER BASICS.

Author: Katie Francis provides technical writing, editing, and project management for engineers and coach engineers to become better technical writers.
With 20 years of experience in engineering seismology with expertise in ground-motion analysis, seismology, quality assurance, and technical writing and editing we are excited to share some of Katie’s experience and knowledge.
Nuclear energy is a substantial contributor to the United States energy grid. Without it, our nation could be in the dark, and I mean that in the most literal sense. The U.S. Energy Information Administration (EIA) reports that nuclear power plants produced 775 billion kilowatt hours (kWh) of electricity in 2023. Given an average annual household electricity usage of 10,791 kWh, nuclear energy generates enough electricity to power about 71.8 million homes for a year. In terms of percentages, nuclear energy accounts for about 20% of the nation’s energy, and it has been that way since 1990 (EIA).
Categorically, nuclear energy ranked third out of the three major types of energy production in 2023. Fossil fuels account for 60% of the total annual energy production in the United States. Renewable sources account for 21%; nuclear energy ranks third at 19%.
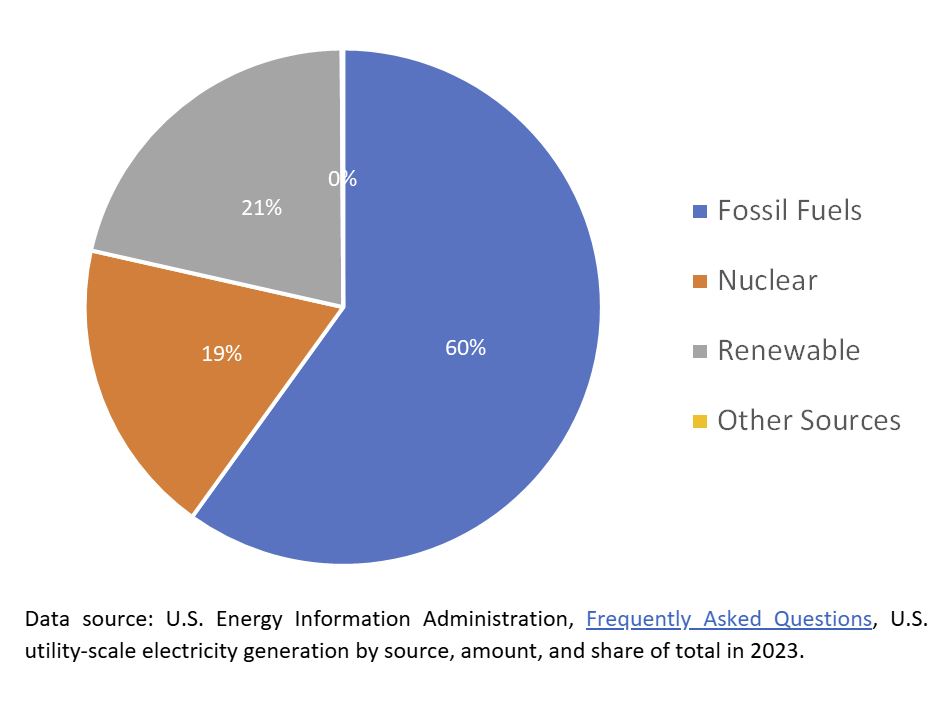
But, if broken down into individual contributors, we see a different picture entirely.
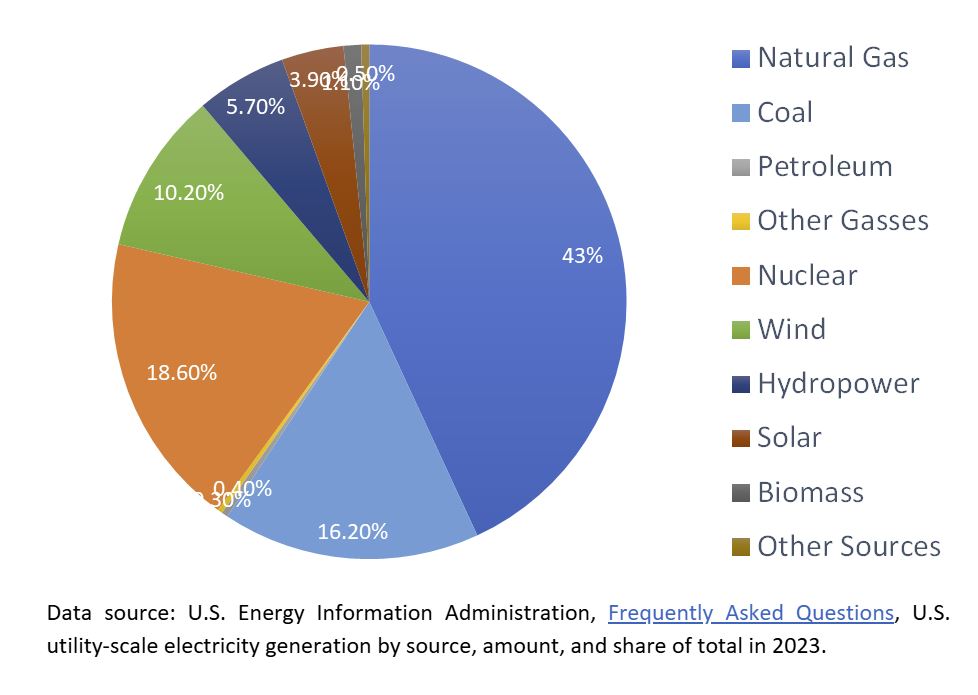
At about 19% of the total annual contribution to the nation’s power grid, nuclear energy ranked second behind natural gas, with coal coming in a close third. Considering coal and natural gas are fossil fuels, nuclear energy is the energy grid’s most significant non-fossil fuel annual contributor. And like renewable energy sources, nuclear energy is clean, meaning it is an energy source that releases no carbon dioxide (CO2) or other greenhouse gasses into the atmosphere. It’s also on par with all the renewable energy sources combined in terms of generation.
In the years ahead, our society will grapple with decisions regarding clean and sustainable energy sources. A 2023 Pew Research Center poll found that most Americans want to increase energy production from clean and renewable sources, and 31% want to phase out fossil fuels entirely, a feat that is currently not possible given the demands on our system and the fact that fossil fuels still account for 60% of our total electricity consumption.
To illustrate this point, let’s consider electric vehicle (EV) usage. An Experian Automotive Trends report from the third quarter of 2024 finds there are about 4.2 million registered electric cars on the road in the United States, and the U.S. Department of Energy reports the National Renewable Energy Laboratory’s projection that there will be 33 million EVs on the road by 2030. How much electricity will it take to power all these cars?
An average EV consumes 4,725 kWh annually, based on Edmonds Research that cites 0.35 kWh per mile for the average EV and 13,500 miles as the national average for miles driven yearly. If there are 4.2 million EVs on the road, it takes almost 20 billion kWh a year to power EVs. If that number increases to 33 million in 2030, it will take 155.9 billion kWh a year. Where will this energy come from?
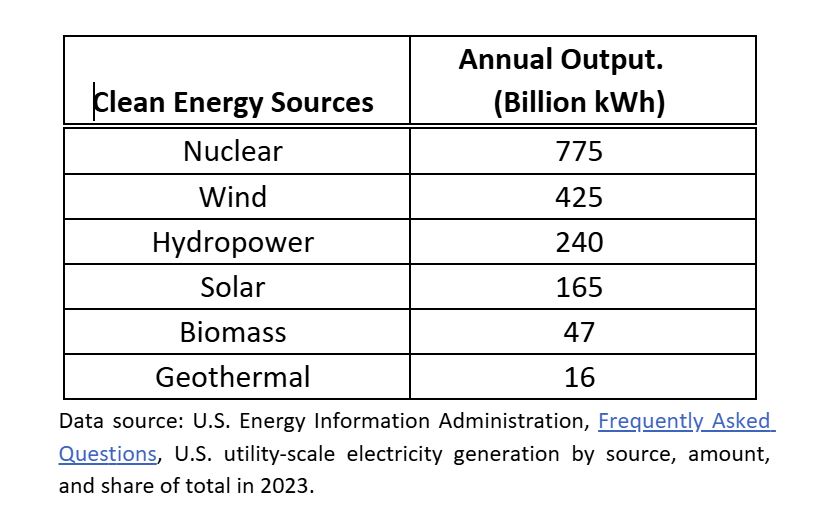
Geothermal and biomass combined are insufficient for powering the future EV load on the system, and solar is pushing it. But this doesn’t take into account any other demand. The United States Census Bureau reported 145,344,626 housing units in the United States in July of 2023. Using an average annual electricity demand from homes of 10,791 kWh, housing alone places a 1.6 trillion kWh load on the system, accounting for nearly all of the clean energy production.
Clean energy sources generate 40% of our energy, and this is barely enough to cover electricity use in the home. The U.S. Energy Information Administration’s report finds that the total residential energy consumption in the U.S. was 38.4% in 2022. If we don’t have enough clean energy to power both our cars and our homes, we have a long way to go if the goal is complete elimination of fossil fuels.
Future energy decisions will be difficult because they require weighing cleanliness against reliability and annual kWh production. As the largest source of clean energy, we must educate ourselves about nuclear power – the benefits, the hazards, and the future potential – when making responsible energy choices.

